CO2-Eating Rocks
Air Date: Week of March 13, 2009

An ultramafic rock deposit in the northern mountains of Oman. (Courtesy of Juerg Matter)
One geologist thinks he has the answer for storing massive amounts of carbon dioxide: turn it into the stuff of seashells. The idea is to use a rock found in the Earth that absorbs the greenhouse gas. Host Bruce Gellerman speaks with scientist Juerg Matter of Columbia University’s Earth Institute.
Transcript
GELLERMAN: Carbon dioxide has the planet between a rock and a hard place - we get needed energy from fossil fuels, yet burning them produces a greenhouse gas that’s causing climate change.
But perhaps the answer lies in the problem: put the gas between a rock and a hard place. Not just any rock - but a type called ultramafic.
Juerg Matter has investigated this ultra-interesting rock. He’s an Associate Research Scientist at the Lamont Doherty Earth Observatory.
Hi Mr. Matter!
MATTER: Thanks for having me.
GELLERMAN: Tell me about ultramafic rock.
MATTER: Yeah, ultramafic rocks are mantle rocks which are usually 25 to 30 miles below surface, and they are rich in magnesium silicate minerals. And actually these magnesium silicate minerals can be used for carbon sequestration. The magnesium is used to carbonate the CO2 into magnesium carbonate minerals.
GELLERMAN: Which is like chalk and limestone, right?
MATTER: Exactly.
GELLERMAN: So it sequesters the carbon dioxide. It changes it.
MATTER: Yeah, that’s true. It changes, you know, the carbon dioxide, which is a gas, into a mineral, which is stable and environmentally benign.

Juerg Matter at the Belvedere Mountain mining site in Vermont (courtesy Sam Krevor)
GELLERMAN: So this would provide, then, a stable sequestration. You wouldn’t have to worry about the gas escaping from a hole in the ground because it wouldn’t be a gas anymore.
MATTER: Exactly. And you know, that’s the big, big advantage in this sequestration option that you produce an environmentally benign calcium or magnesium carbonate mineral, which cannot leak back CO2 back into the atmosphere.
GELLERMAN: Well, how long does this chemical reaction take in nature?
MATTER: Every day it takes place, but it’s, as we could see in Oman on average, you know, these carbonates, you can find in these types of rocks are 26,000 years old. So it’s on geologic time, much faster than we thought before, but it’s not fast enough for our engineered process to really, you know, soak up a lot of carbon dioxide.
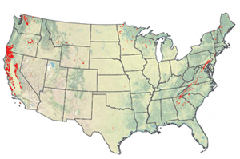
Ultramafic rocks (in red) that potentially could absorb CO2 (courtesy U.S. Geological Survey)
GELLERMAN: So how can we speed up this chemical reaction between the ultramafic rock and CO2?
MATTER: Generally you can grind the rocks to really fine powder. And then you can react the rocks with carbon dioxide on the surface here, like in a cement factory and produce, you know, these carbonate minerals. But you can also think about to inject CO2 into these rocks. And also if you heat up the rocks to 185 degrees, the reaction just takes off and it goes forever, so it’s sustainable. And so, heat speeds up the reaction.
GELLERMAN: Now, we don’t have to drill down, you know, 45 miles into the Earth to do this, do we? Or do we?
MATTER: Oh no, no, no. These rocks are, you know, through tectonic processes these rocks were thrust onto the continental crust because of the mountain forming processes put these rocks on the surface.
GELLERMAN: Well you mention that you can find this type of rock in Oman. Where in the United States is this rock found?

An ultramafic rock deposit in the northern mountains of Oman.
(Courtesy of Juerg Matter)
MATTER: Yeah, in the United States you find, you know, a lot of ultramafic rocks in California, Oregon and Washington State. You will find these rocks also in, you know, along the whole Appalachian Mountain belt. And you also find it in the interior, in Wyoming, Montana, and Minnesota. And a little bit, you know, excuse me in Texas and in the South.
GELLERMAN: How much CO2 could we absorb? I mean we’re pumping out like 30 billion tons a year of the stuff.
MATTER: Yeah, exactly. I mean, you could sequester, you know, the next 500 years of U.S. CO2 emissions. For Oman, itself, and in Oman we have an ultramafic rock body of 350 kilometers long, 40 kilometers wide and 5 kilometers thick. If we would use every magnesium ion in these rocks and convert that to carbonates we would have thousands of years absorption capacity. But, you know, more realistic, is roughly, you know, on a scale of one billion tons of CO2 per year – that’s possible in this type of rocks.
GELLERMAN: So if we had an international trading scheme, where there was really value on CO2, countries that had this type of rock, could make money out of it.
MATTER: Exactly. And that’s why, you know, the Ministry of Commerce of the Sultanate of Oman is interested. You know, they have the biggest ultramafic rock body in Oman and it’s just a black green rock sitting there, but you know it could be used as a source of income.
GELLERMAN: Juerg Matter is an Associate Research Scientist at Lamont Doherty Earth Observatory, part of the Earth Institute at Columbia University. Mr. Matter thanks a lot.
MATTER: Yeah, thanks a lot. It was good talking to you.
Links
Living on Earth wants to hear from you!
Living on Earth
62 Calef Highway, Suite 212
Lee, NH 03861
Telephone: 617-287-4121
E-mail: comments@loe.org
Newsletter [Click here]
Donate to Living on Earth!
Living on Earth is an independent media program and relies entirely on contributions from listeners and institutions supporting public service. Please donate now to preserve an independent environmental voice.
NewsletterLiving on Earth offers a weekly delivery of the show's rundown to your mailbox. Sign up for our newsletter today!
 Sailors For The Sea: Be the change you want to sea.
Sailors For The Sea: Be the change you want to sea.
 The Grantham Foundation for the Protection of the Environment: Committed to protecting and improving the health of the global environment.
The Grantham Foundation for the Protection of the Environment: Committed to protecting and improving the health of the global environment.
 Contribute to Living on Earth and receive, as our gift to you, an archival print of one of Mark Seth Lender's extraordinary wildlife photographs. Follow the link to see Mark's current collection of photographs.
Contribute to Living on Earth and receive, as our gift to you, an archival print of one of Mark Seth Lender's extraordinary wildlife photographs. Follow the link to see Mark's current collection of photographs.
 Buy a signed copy of Mark Seth Lender's book Smeagull the Seagull & support Living on Earth
Buy a signed copy of Mark Seth Lender's book Smeagull the Seagull & support Living on Earth